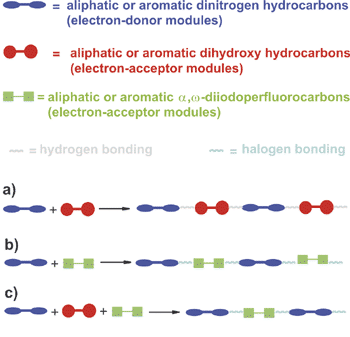
The term halogen bonding describes the tendency of halogen atoms to attractively interact with lone pair possessing atoms. The binding features and structural properties of halogen bonding are discussed and applied to drive the intermolecular self-assembly of hydrocarbons and perfluorocarbons in chemo-, site-, and enantioselective supramolecular syntheses. The halogen bonding is thus an effective and reliable tool in crystal engineering at the disposal of the supramolecular chemist.