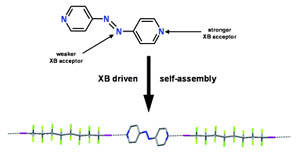
The halogen-bonding-driven self-assembly of α,ω-diiodoperfluoroalkanes with trans-4,4-azobipyridine gives rise to infinite 1D networks in the crystalline state. The four nitrogen atoms of the azobispyridine module could allow for the formation of various isomeric products. Interestingly, a site-selective supramolecular synthesis occurs, since only the pyridyl nitrogen atoms are involved in the recognition process. This selectivity can be rationalized on the basis of a higher basicity and steric accessibility of the pyridine nitrogens, with respect to the azo nitrogens, and with the formation of an architecture where the hydrocarbon and perfluorocarbon modules segregate.