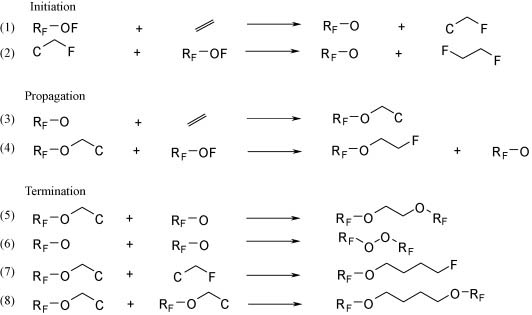
In the reaction between perfluoroolefins and perfluoroalkylhypofluorites the existence of two different free radical reaction mechanisms is demonstrated by the presence of characteristic byproducts. These kinetic schemes can be experimentally controlled by tuning the hypofluorite concentration in the reaction media. In particular, in the reactions between trifluoromethyl hypofluorite and highly reactive perfluoroolefins like CF2=CFOCF3 and CF2=CF2, the free radical oligomerization and dimerization products can be suppressed by utilizing the opportune experimental conditions. The pure perfluorinated ethers obtained, having low Ostwald coefficient, can be utilized as contrast agents for diagnostic ultrasound imaging injectable microbubbles composition.