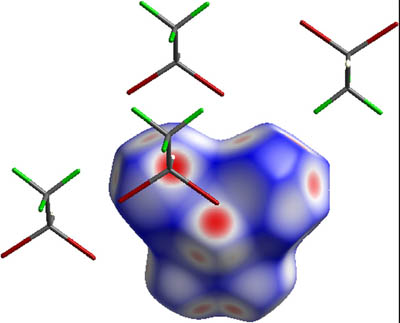
Intermolecular interactions and the role of fluorine substitution have been investigated for a
halogenated-ethane anesthetic. 2-Bromo-2-chloro-1,1,1-trifluoroethane, BrClCHCF3 (Halothane), has
been in situ pressure frozen in a diamond anvil cell and its structure determined by single-crystal X-ray diffraction at 1.85(5) GPa/296 K. Crystal is triclinic, space group P1. In this racemic structure the enantiomorphic molecules are substitutionally disordered at the same general positions in that way that bromine and chlorine atoms occupy the same site at the 50:50 ratio. Despite the fact that only the Br and
Cl atoms are disordered, the crystal packing is dominated by halogen...halogen and halogen...hydrogen interactions. This X-ray diffraction study provides structural explanation of considerably increased vaporpressure of Halothane compared to its hydrogenated analogue.