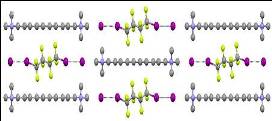
Halogen bonding has increasingly facilitated the assembly of diverse host-guest solids. Here, we show that a well-known class of organic salts, bis(trimethylammonium) alkane diiodides, can reversibly encapsulate α,ω-diiodoperfluoroalkanes (DIPFAs) through intermolecular interactions between the host's I- anions and the guest's terminal iodine substituents. The process is highly selective for the fluorocarbon that forms an I-...I(CF2)mI...I- superanion that is matched in length to the chosen dication. DIPFAs that are 2 to 12 carbons in length (common industrial intermediates) can thereby be isolated from mixtures by means of crystallization from solution upon addition of the dissolved size-matched ionic salt. The solid-state salts can also selectively capture the DIPFAs from the vapor phase, yielding the same product formed from solution despite a lack of porosity of the starting lattice structure. Heating liberates the DIPFAs and regenerates the original salt lattice, highlighting the practical potential for the system in separation applications.