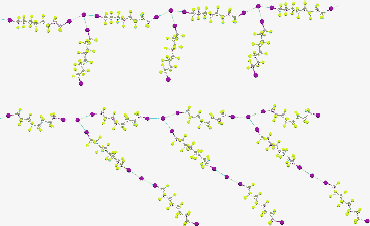
Self-assembly of ternary mixtures composed of cation segregating agent/inorganic halide (penta-tert-butyl-pentakis(ethoxycarbonylmethoxy)calix[5] arene (1)/NaI or 4,7,13,16,21,24-hexaoxa-1,10-diazabicyclo[8,8,8]hexacosane (4)/BaI2) and diiodoperfluorocarbon (1,8-diiodoperfluorooctane (2)) produces crystalline supramolecular salts 3 and 5, respectively, whose structural characteristics in the solid state have been elucidated by single-crystal X-ray analyses. Encapsulation of alkali or alkaline-earth metal ions inside the complexing pocket of the appropriate receptor generates naked iodide anions, which effectively drive the self-assembly of diiodoperfluorooctane 2 into halogen-bonded supramolecular anions with a comb-like copolymer structure.