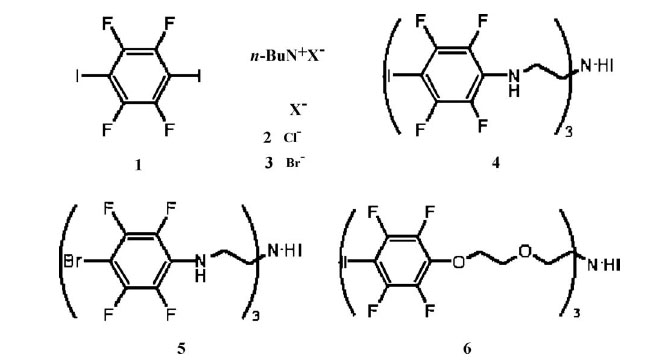
The supramolecular organization in six solid assemblies involving iodo- and bromoperfluoroarene
derivatives is described. Single crystal X-ray analyses show that the formation of the supramolecular architectures is controlled by I-...Br?ArF, I-...I?ArF, Br-...I?ArF, and Cl-...I?ArF halogen bondings thus proving the X-...X'?ArF supramolecular synthon, where X can be the same as or different from X', is particularly robust. In five of the described architectures halide anions form two halogen bondings and form infinite chains wherein dihaloperfluoroarenes, which function as bidentate electron acceptors, and halide anions, which function as bidentate electron donors, alternate. This behaviour shows halide anions have a fair tendency to work as bidentate halogen bonding acceptors.