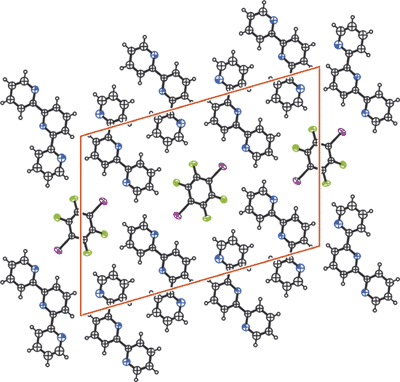
2,2':6',2''-Terpyridine is a well-known electron donor module in metal coordination chemistry and typically works as a tridentate ligand. Here it is shown to work also as an electron donor towards iodoperfluorocarbons both in liquid and solid phases. Halogen-bonded supramolecular systems are thus obtained. Specifically, the terpyridine self-assembles with 1,2,4,5-tetrafluoro-3,6-diiodobenzene and affords the trimeric adduct, which is stable and crystalline in the air at room temperature. Single crystal X-ray analysis shows how in the co-crystal both the iodine atoms of the same diiodotetrafluorobenzene molecule are halogen-bonded to the nitrogen atoms of external pyridine rings of two different terpyridine molecules that act as monodentate electron donors.