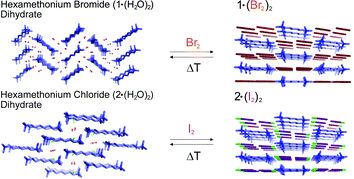
1,6-Bis(trimethylammonium)hexane bis(trihalides) and mixed bis(trihalides) have been synthesized by treating the corresponding dihydrated halides with molecular dihalogens under gas?solid and solution conditions. Despite the starting halides being non-porous, the trihalide syntheses occur homogeneously, in quantitative yields, and reversibly. In all cases halogen bond prevails over hydrogen bond, dihalogens substitute for the hydration water of starting halide anions and trihalides are formed. The stability of the obtained trihalides is mainly due to cooperative halogen bond and cation templation effect. Hexamethonium halides are proven effective solids for the clathration and storage of molecular dihalogens. While the starting salts are not isostructural, all the formed trihalides and mixed trihalides are isostructural.