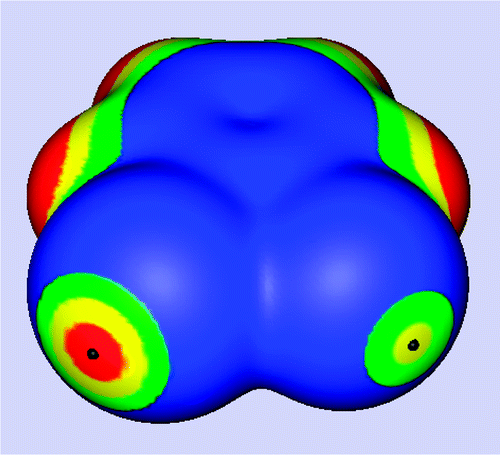
Because of the anisotropies of their electronic charge distributions, many covalently bound halogen atoms have regions of positive electrostatic potential (positive σ-holes) on their outer portions. Through these, the halogens can interact attractively with negative sites. It has sometimes been questioned whether fluorine, the least polarizable halogen, can form halogen bonds, especially in solids. Here we present computational and crystallographic evidence demonstrating that it can indeed do so, in both the gaseous and the solid phases. We show computationally, through a series of examples, that fluorine can have positive σ-holes when linked to strongly electron-withdrawing residues and that it can interact with Lewis bases to form gas phase halogen-bonded complexes. Through statistical analyses of data from the Cambridge Structural Database, we demonstrate that such fluorines do also halogen bond in the solid state, and we show several specific cases of this.