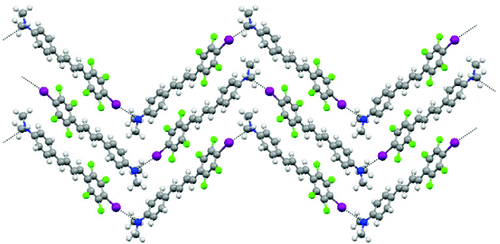
Three new push pull molecules having either pyridyl or N, N-dimethylanilino groups as electron-donor moieties and the p-iodotetrafluorophenyl ring as an electron-acceptor group have been synthesized and their single crystal X-ray structures are reported. Halogen bonding drives the self-assembly of these molecules in the solid state giving rise to head-to-tail halogen-bonded infinite polar chains which crystallize in an antiparallel arrangement. The three new nonlinear optical (NLO)-phores synthesized show high hyperpolarizabilities at the molecular level in solution. Rationalization of the obtained NLO measurements is supported by molecular modeling calculations.