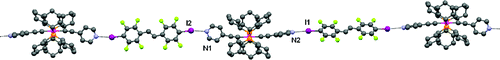
The synthesis and characterization of low k one-dimensional (1D) hybrid organic?organometallic supramolecular ribbons 3a,b, through halogen-bond driven co-crystallization of trans-[Pt(PCy3)2(C≡C-4-py)2] (1) with 1,4-diiodotetrafluorobenzene (2a) and trans-1,2-bis-(2,3,5,6-tetrafluoro-4-iodophenyl)-ethylene (2b), are reported. The co-crystals 3a,b have been obtained by isothermal evaporation of a chloroform solution containing the corresponding starting materials at room temperature. X-ray structure determinations show that noncovalent interactions other than halogen bonds help in the construction of the crystal packing; these interactions are stronger in 3b, thus reducing the chain mobility with respect to 3a. Accordingly, the broadband dielectric spectroscopic determinations, carried out from 10?2 to 107 Hz and at a temperature ranging from 25 to 155 °C, showed that both 3a and 3b materials exhibit a real component of dielectric permittivity (ε′) significantly lower than SiO2. In particular in the case of 3b, the rigidity of the 1D chain explains the observed ε″ and tan δ values. A permittivity value that is significantly lower than that of the silica reference, tan δ values lower than 0.02 in the entire investigated temperature range, and less than 0.004 at T < 100 °C make 3b a very promising low k hybrid organic?organometallic material for application as dielectric films in next generation microelectronics.