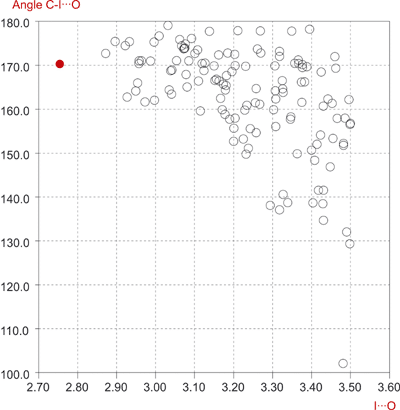
The O···I halogen bonding between 4,4?-dipyridyl N,N?-dioxide and 1,4-diiodotetrafluorobenzene (2.75 Å) is the shortest reported to now in the crystallographic literature. This short interaction is the result of our careful crystal engineering that allows the characteristics of the oxygen-iodine interaction to be established in details.
Indeed, the higher the electron density on the electron donor site, the stronger its electron donor ability when halogen bonding is formed. This heuristic principle allowed us to design and realise oxygen donors that are stronger than the nitrogen ones in solid and liquid phases.