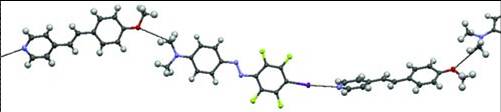
It is demonstrated that halogen bonding can be used to construct low-molecular-weight supramolecular complexes with unique light-responsive properties. In particular, halogen bonding drives the formation of a photoresponsive liquid-crystalline complex between a non-mesogenic halogen bond-donor molecule incorporating an azo group, and a non-mesogenic alkoxystilbazole moiety, acting as a halogen bond-acceptor. Upon irradiation with polarized light, the complex exhibits a high degree of photoinduced anisotropy (order parameter of molecular alignment > 0.5). Moreover, efficient photoinduced surface-relief-grating (SRG) formation occurs upon irradiation with a light interference pattern, with a surface-modulation depth 2.4 times the initial film thickness. This is the first report on a halogen-bonded photoresponsive low-molecular-weight complex, which furthermore combines a high degree of photoalignment and extremely efficient SRG formation in a unique way. This study highlights the potential of halogen bonding as a new tool for the rational design of high-performance photoresponsive suprastructures.