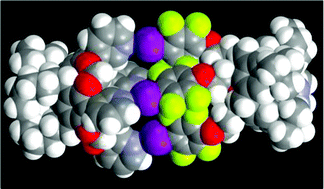
A halogen-bonded capsule is obtained via directed assembly of a rigid tetra(3-pyridyl) cavitand and a flexible tetra(4-iodotetrafluorophenyl)calix[4] arene. The pyridyl nitrogen atoms from one cavitand molecule interact with the iodine atoms of a single calixarene molecule through short and directional I⋯N halogen bonds. The flexibility of the ethylenedioxy moieties on the calixarene platform results in positional flexibility of the iodotetrafluorobenzene sites which, coupled with a supramolecular chelating effect, allow for an effective partner-induced geometric fitting between four nitrogen atoms on the cavitand and four iodine atoms on the calixarene.