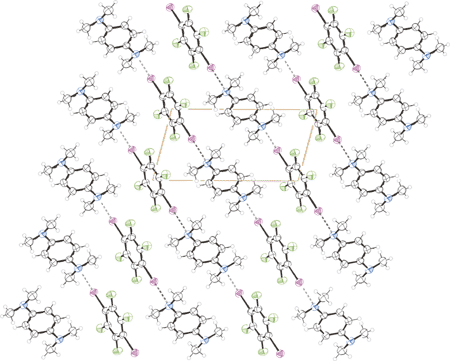
 '-tetramethyl-p-phenylene-diamine and 1,4-diiodotetrafluorobenzene. Dashed lines represent the halogen bonding between the PFC and HC modules. Colours are as follows: black, carbon and hydrogen; blue, nitrogen; violet, iodine; green, fluorine.'>
'-tetramethyl-p-phenylene-diamine and 1,4-diiodotetrafluorobenzene. Dashed lines represent the halogen bonding between the PFC and HC modules. Colours are as follows: black, carbon and hydrogen; blue, nitrogen; violet, iodine; green, fluorine.'>
N,N,N?,N?-tetramethyldianilines are well known donor modules tailored to π,π-interaction driven self-assembly processes. When N,N,N?,N?-tetramethyl-1,4-phenylenediamine and bis[4-(N,N-dimethylaminophenyl)]methane interact with 1,4-diiodotetrafluorobenzene, the halogen bonding organises the perfluorocarbon and hydrocarbon modules into one dimensional linear networks, overcoming the low affinity between the perfluorocarbon and hydrocarbon modules and their tendency to give π,π stacks. The general effectiveness of N,N,N?,N?-tetramethyldianilines as specifically tailored telechelic modules in the exo-recognition of dihaloperfluorocarbons has been demonstrated.