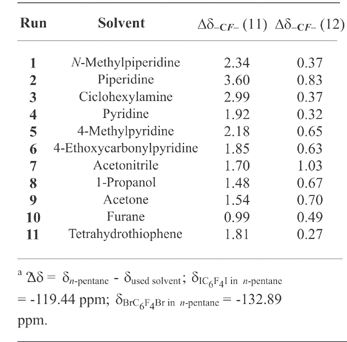
The halogen bonding between structurally different halo-perfluorocarbons (Rf - X, Rf = aliphatic or aromatic perfluorinated residue, X = I, Br, Cl) and heteroatom containing hydrocarbons (HC-D, HC = hydrocarbon residue, D = electron donor heteroatom) results in the formation of Rf - X···D-HC complexes in the liquid phase. This formation strongly affects
the 19F NMR spectra of the perfluorinated partners. The Δδ-CF2X and the Δδ-CF=CX values of perfluoroalkyl and aryl derivatives is proven to be a simple, powerful, and versatile tool to rank the tendency of perfluorocarbon and hydrocarbon modules to take part in the formation of halogen bonds.
the 19F NMR spectra of the perfluorinated partners. The Δδ-CF2X and the Δδ-CF=CX values of perfluoroalkyl and aryl derivatives is proven to be a simple, powerful, and versatile tool to rank the tendency of perfluorocarbon and hydrocarbon modules to take part in the formation of halogen bonds.