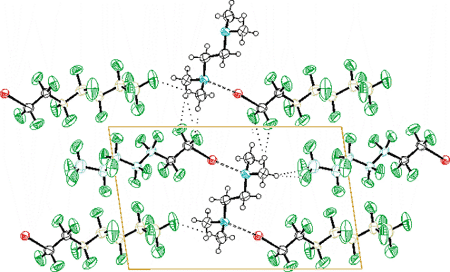
 -tetramethylethylene-diamine and 1-iodoperfluoroheptane. Colours are as follows: black, carbon and hydrogen; blue, nitrogen; red, iodine; green, fluorine. Dotted lines represent weak H···F hydrogen bonds. Dashed lines represent N···I halogen bonds.'>
-tetramethylethylene-diamine and 1-iodoperfluoroheptane. Colours are as follows: black, carbon and hydrogen; blue, nitrogen; red, iodine; green, fluorine. Dotted lines represent weak H···F hydrogen bonds. Dashed lines represent N···I halogen bonds.'>
1-Iodoperfluoroheptane and tetramethylethylenediamine (TMEDA) form a 2:1 ratio stable aggregate. A similar behaviour is shown by several other 1-iodoperfluoroalkanes and arenes. These aggregates have been characterised in solution by 1H / 19F-NMR spectroscopy and in the solid state through IR and single crystal X-ray diffraction. The determined structure of the cocrystal between TMEDA and 1-iodoperfluoroheptane shows the second shortest N···I interaction found in the crystallographic literature [2.762(3) Å] and the interdigitation of perfluorocarbon and hydrocarbon modules due to co-operative -C-H···F-C- interactions. Calculations with the aim to quantify the strength of these latter interactions have been also performed.