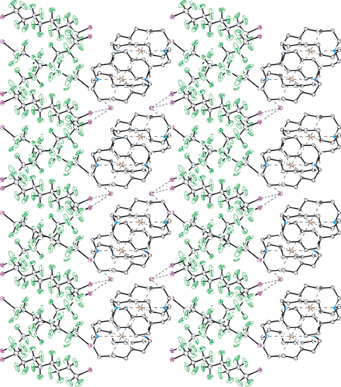
The strong halogen bonding occurring between naked iodide ions and iodoperfluoroalkanes can overcome the low affinity that hydrocarbon derivatives and inorganic salts have for perfluorinated derivatives, resulting in the compatibilization of otherwise immiscible compounds. Small amounts of iodoperfluoroalkanes boost the solubility of inorganic salts in fluorinated solvents. In the solid phase, the halogen bonding between the iodide ion of cryptated salts and differently sized diiodoperfluoroalkanes allows the design and obtainment of three-component mixed supramolecular architectures. Segregation phenomena and halogen bonding features control the formation of new crystalline materials containing fluorous layerlike and interpenetrated networks.