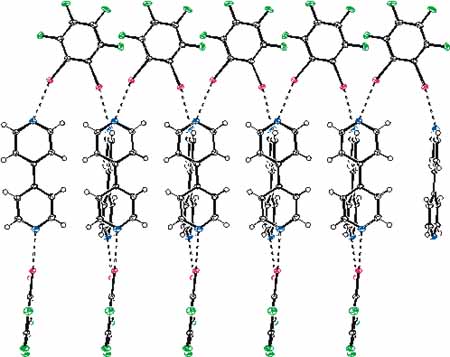
The N···Br halogen bonding drives the self-assembly of 1,4-dibromo-tetrafluorobenzene and its 1,3- or 1,2- analogues with various dipyridyl derivatives. Several isomeric supramolecular architectures have been obtained as co-crystals which are stable in the air at room temperature. The solid-state features of these 1D infinite chains have been fully characterised by single crystal X-ray, Raman, and IR analyses. The occurrence of N···Br halogen bonding in solution has been detected by 19F NMR. The N···Br halogen bonding is highly selective and directional and the geometry of the single strands of non-covalent co-polymers is controlled by the geometry of halogen bonding donor and acceptor sites on starting modules. The composition and topology of the instructed networks can be predicted with a great accuracy. Experiments on competitive co-crystal formation established the relative strength of the N···Br interaction compared to other halogen bondings and the tendency of different modules to be involved in site-selective supramolecular syntheses.