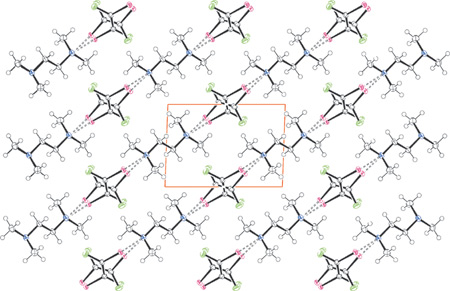
 -tetramethylethylene-diamine and trans-1,2-diiododifluoroethene. Dashed lines represent the halogen bonding between the PFC and HC modules. Colours are as follows: Black, carbon and hydrogen; blue, nitrogen; violet, iodine; green, fluorine.'>
-tetramethylethylene-diamine and trans-1,2-diiododifluoroethene. Dashed lines represent the halogen bonding between the PFC and HC modules. Colours are as follows: Black, carbon and hydrogen; blue, nitrogen; violet, iodine; green, fluorine.'>
N···I Halogen bondings drive the intermolecular recognition between (E)-1,2-diiodo-1,2-difluoroethene, which works as a bidentate electron acceptor, and TMEDA or 4,4'-dipyridyl, which work as bidentate electron donors. The presence of 1D infinite chains in both co-crystals has been established through X-ray analyses.