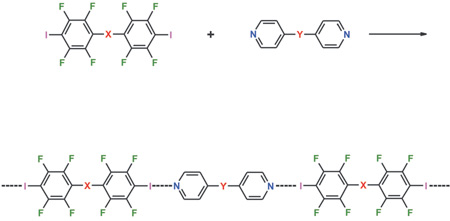
The SNAr between oxygen or nitrogen nucleophiles and iodopentafluorobenzene is a versatile modular approach for the synthesis of telechelic á,ù-di-(2,3,5,6-tetrafluoro-4-iodophenyl) derivatives. Despite the presence of a +M substituent in the para position to iodine, these derivatives work as effective halogen bond donors and form strong I···N interactions with telechelic á,ù-di-(4-pyridyl) derivatives. In the resulting 1D infinite chains the starting tectons alternate in an almost collinear fashion. The detailed structure of some of the obtained adducts was established through X-ray analyses. The overall structural pattern of these co-crystals is largely independent of the starting tectons size. The simple variation of the length of the connecting modules in the resulting aggregates can be considered as a sort of metric engineering.