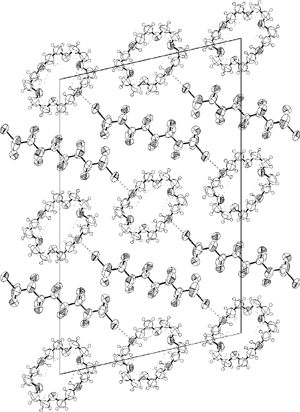
Both the nitrogen atoms of Kryptofix® 2.2. work as electron-donors to the iodine atoms of α,ω-diiodoperfluoroalkanes giving rise to infinite 1D chains as white and crystalline solids. The structural features of these non-covalent co-polymers have been studied using various techniques (IR, 1H-NMR, 19F-NMR, X-ray). It has been established that, once halogen bonded to perfluoroalkyliodides, Kryptofix® 2.2. adopts the same conformation as in the pure form, namely the exo-exo topology. The co-existence of halogen-bonding and hydrogen-bonding in the obtained supramolecular architectures allows pre-organisation of the interacting partners to be pursued as a new strategy in the rational design of divergent self-assembly processes.