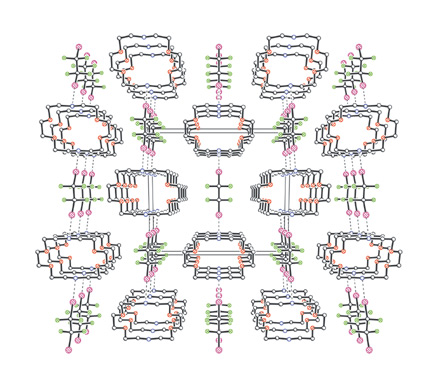
The formation of infinite 1D networks wherein diiodoperfluorocarbons are halogen bonded to di-nitrogen substituted hydrocarbons is described. The N···I non-covalent interaction is specific, directional, and strong enough to effectively overcome the low affinity between perfluorocarbon and hydrocarbon modules and to drive their self-assembly in the solid and liquid phase. The effectiveness of the interaction is largely independent from the overall structure of the involved modules. Indeed, supramolecular architectures have been obtained starting from diiodoperfluoroalkanes and ?arenes (electron poor motifs) as well as from pyridine derivatives, and di- or trialkylamines (electron rich motifs). The halogen bonding can now be considered as a first choice intermolecular interaction in crystal engineering