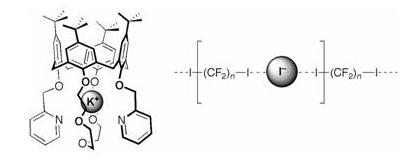
1,3-Bis(alfa-picolyloxy)-p-tert-butylcalix[4]crown-5 in the cone conformation (2), 1,8-diiodoperfluorooctane or 1,6-diiodoperfluorohexane, and potassium iodide ternary mixtures undergo in solution self-sorting and afford crystalline ?supramolecular salts?. These hybrid materials consist of supercation [K+ < 2] and superanion [I-(CF2)n-I???I????I-(CF2)n-I???I????)] (n = 6, 8) components. In the supercations the potassium ion is embedded in the ionophoric pocket created by the heteroatoms present at the lower rim. In the superanions the iodide ions form infinite fluorous polyanionic chains as a result of a self-assembly process which relies on halogen bonding. Both cation encapsulation and anion-perfluorocarbon halogen bonding were detected in solution by 1H and 19F NMR, and in the gas phase by ESI MS.