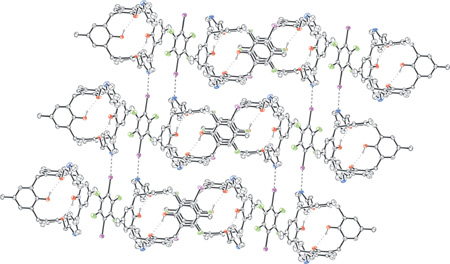
Attractive ð,ð-interactions between two of the four outside cavity faces of 1,3-bis-pyridilcalix[4]arene and both faces of 1,4-diiodotetrafluorobenzene form infinite 1D non-covalent ribbons where the two modules alternate. These ribbons are cross-linked by electron donor-acceptor interactions between picolyl nitrogen atoms of the calixarene in one chain and the iodine atoms of perfluoroarene in another chain forming the 2D supramolecular network. A similar behaviour is also shown by 1,4-dibromotetrafluorobenzene. The ability of electron poor arenes to elicit the exo-receptor potential of calixarene module by connecting their outside faces via ð,ð-interactions may be developed as a new and general binding protocol in calixarene self-assembly processes.