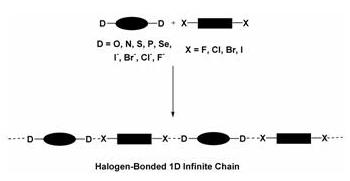
Halogen bonding is the non-covalent interaction between halogen
atoms (Lewis acids) and neutral or anionic Lewis bases1. The main
features of the interaction will be given and the close similarity with
hydrogen bonding will become apparent. Some heuristic principles
will be presented in order to develop a rational crystal engineering
based on halogen bonding. The focus will be in particular on halogen
bonded supramolecular architectures given by halocarbons and related
structures. The potential of the interaction will be shown by useful
applications in fields as diverse as synthetic chemistry, material
science, and bioorganic chemistry.