|
Halogen Bonding versus Hydrogen Bonding in Driving Self-Assembly Processes |
|
Angew. Chem. Int. Ed. 2000, 39, 1782
|
|
E. Corradi, S. V. Meille, M. T. Messina, P. Metrangolo, G. Resnati |
|
|
|
|
|
|
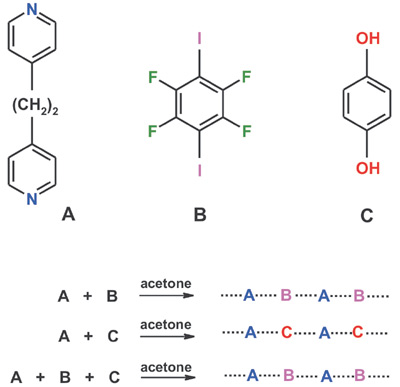
When the three aromatic components A, B, and C are present at the same time in the solution, the halogen bonding drives the preferential A-B intermolecular recognition, and the corresponding solid co-crystal precipitates. The hydrogen bond donor C remains in solution. |
|
|
|
|
|
|
ABSTRACT
We show that when the recognition pattern controlling self-assembly processes can be based on either hydrogen bonding or halogen bonding, the latter dominates over the former and singles out the molecules that will build up the resulting supramolecular architectures.
|
|
|
|
|
