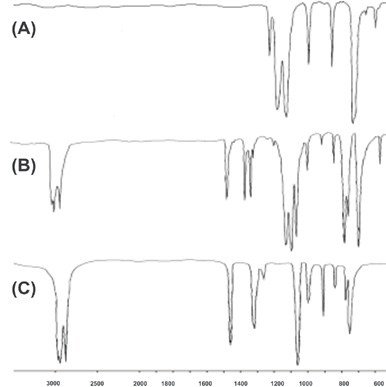
An attractive intermolecular interaction that has been called ?halogen bonding? exists between the nitrogen, sulfur, or oxygen atoms present in HC motifs and the iodine atom of PFC residues. The ?halogen bonding? is strong enough to overcome the low affinity existing between PFC and HC compounds, driving their self-assembly into supramolecular architectures. The non-covalent co-polymer formed by 1,2-diiodotetrafluoroethane with diazabicyclooctane has been prepared and characterized by FT-IR and Raman spectroscopies. We propose the changes shown on the vibrational spectra of single PFC and HC components, once involved in halogen bonded co-polymers, as diagnostic probes of the interaction and as tools to rank the electron-donor ability of differently heteroatom substituted hydrocarbons.