 -4,4'-dipyridil-dioxide and 1,4-diiodotetrafluorobenzene. The strong halogen bondings are dashed, the weak hydrogen bondings are dotted. Colours are as follows: violet, iodine; red, oxygen; blue, nitrogen; green, fluorine; black, carbon and hydrogen.'>
-4,4'-dipyridil-dioxide and 1,4-diiodotetrafluorobenzene. The strong halogen bondings are dashed, the weak hydrogen bondings are dotted. Colours are as follows: violet, iodine; red, oxygen; blue, nitrogen; green, fluorine; black, carbon and hydrogen.'>
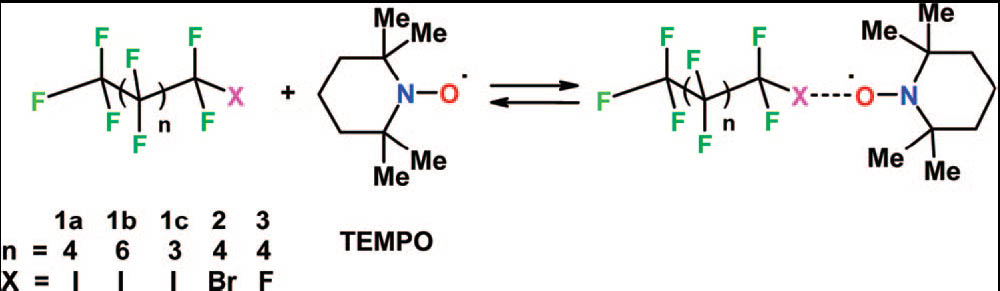
Heteroaromatic N-oxides are shown to work as effective electron donors towards perfluorocarbon iodides. This non-covalent interaction is strong enough to drive the self-assembly of perfluorocarbon and hydrocarbon modules into discrete aggregates or 1D infinite networks. The shortest O···I intermolecular distance reported to now in the crystallographic literature has been obtained. The effectiveness of the O···I-RF halogen bonding with respect to the better studied N···I-RF interaction is discussed.